Supporting an international oncology company to enhance their global quality approach
5 min read 23 July 2025
A multinational oncology company wanted to improve its approach to Good Clinical Practice (GCP) quality across its Global Clinical Operations (GCO). They needed to do this to support their ambitious global R&D pipeline expansion plans outside of their primary market, which would require a stronger approach to quality in order to compete and satisfy customers in new markets.
The organisation had three requirements:
- Shift mindsets within GCO from a reactive, tactical approach, to a proactive quality culture where quality is embedded in day-to-day activities
- Design a Global GCP Quality framework that effectively connected strategy, governance, roles, processes, and systems
- Define clear roles and responsibilities across regions to enable stronger collaboration across GCO
Baringa’s leading expertise in R&D transformation
We were selected as the delivery partner because of our deep expertise in advising global pharmaceutical companies on R&D quality across the pharma value chain. We brought proven experience in designing governance models that support compliance, quality and inspection readiness.
This project also built on our previous work with the client to design an initial GCP quality strategy and inspection readiness approach.
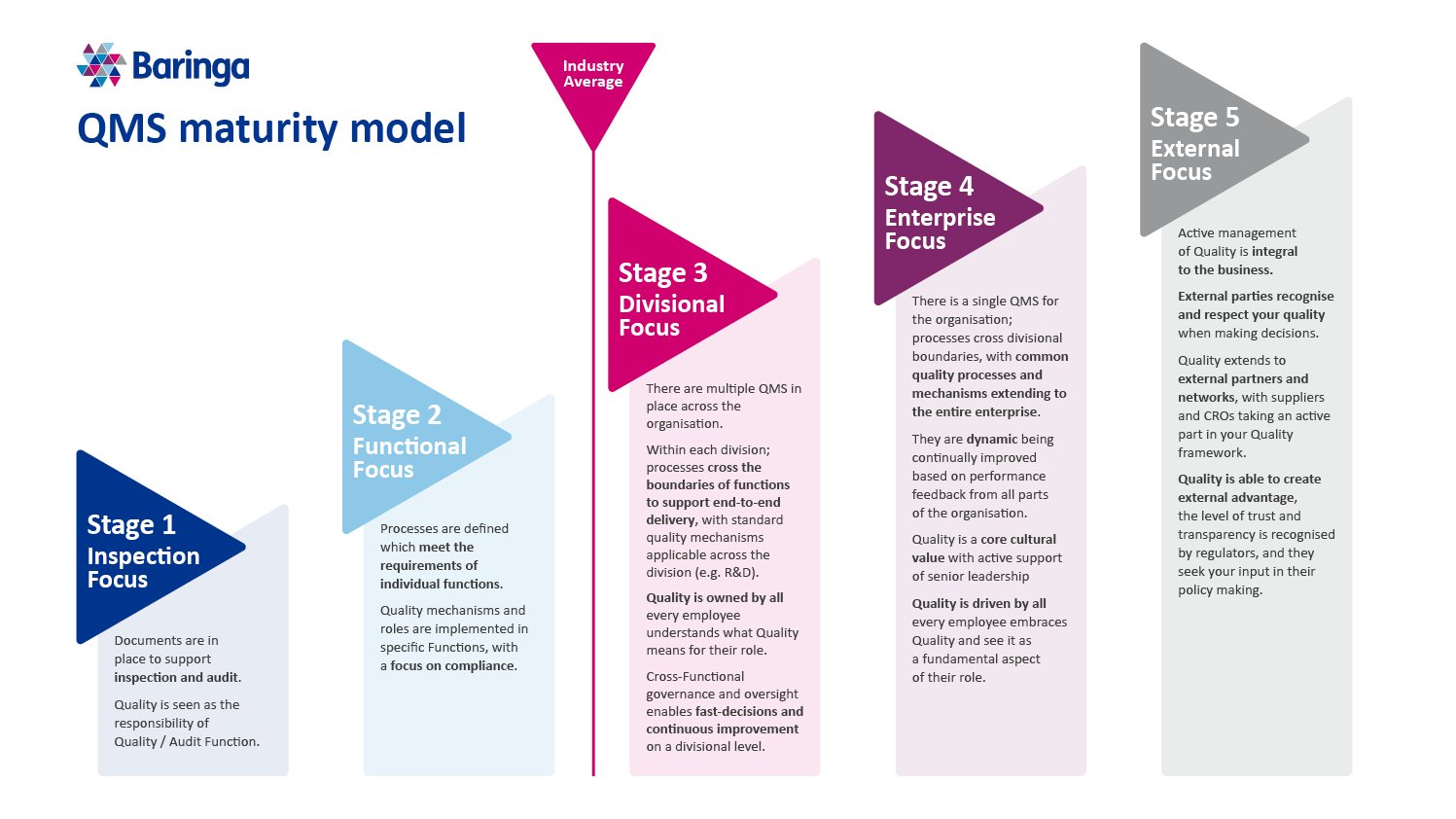
Central to our approach for this engagement was our proprietary Quality Systems maturity model, which helps pharma companies evolve from reactive inspection engagement, through to an enterprise-wide focus on quality, and ultimately to making quality a competitive advantage.
Our work with the client focused on the first step in the maturity model: developing an approach where functional quality policies and processes are embedded across markets.
Our ‘people-led’ approach was important as the GCP Quality Framework needed to be supported within the GCO teams, so the practical benefits of a single, well-defined approach to quality could be realised.
Alongside this work, we were also delivering an R&D process simplification programme, learning & development (L&D) initiatives and a change management approach within GCO, allowing us to take a joined-up approach to R&D transformation with the overall intent of increasing quality maturity across the organisation.
Consistent quality approach embedded in the organisation
Over the course of three months, we led the delivery of the GCP Quality Framework. We supported the client to define a vision for the quality framework, in order to set expectations across the organisation about the value the framework would bring. We facilitated global workshops to co-create the framework content.
We also shaped a quality dashboard for the client, to showcase how they could use live reporting to ensure real time monitoring of compliance, quality, and inspection readiness against defined metrics.
We used our tried and tested R&D change management approach to drive adoption of the framework. This involved working side by side with teams across markets to communicate how it should be used, and developing training modules to upskill teams across the organisation.
The resulting framework now serves as a governing document for future quality policies and processes. The framework provides a single, enterprise-wide reference point for GCP quality guidance, which can be evolved over time to support a consistent approach to R&D quality across the business.
"Baringa nailed it! It has been a pleasure working with them to take our first steps towards GCP quality maturity across our business."
Global Quality Lead
Our Experts
Related Insights

AI in healthcare needs more than hype – it needs quality
Digital and AI innovation is rapidly propelling the healthcare and life sciences industry into a new technological era, where the art of the possible is being continuously reimagined, creating more opportunities to unlock value and ultimately drive better health outcomes for patients.
Read more
Reimagining a critical R&D process through AI
A top 10 global biopharmaceutical company partnered with Baringa to address a challenge familiar across R&D.
Read more
Transformation of QMS: Why evolution is inevitable and urgent
The pharmaceutical industry is experiencing a rapid acceleration in the adoption of artificial intelligence (AI), automation and advanced digital technologies across R&D.
Read more
Enabling commercial agility in medical device manufacturing
A global medical device company was operating with limited visibility across its commercial and operational functions.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?