Embedding cultural change at pace in a complex GxP environment
10 min read 20 November 2025
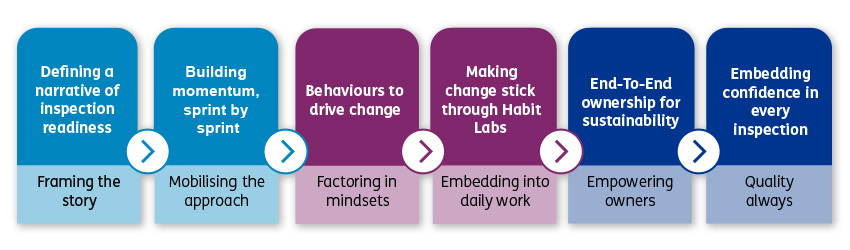
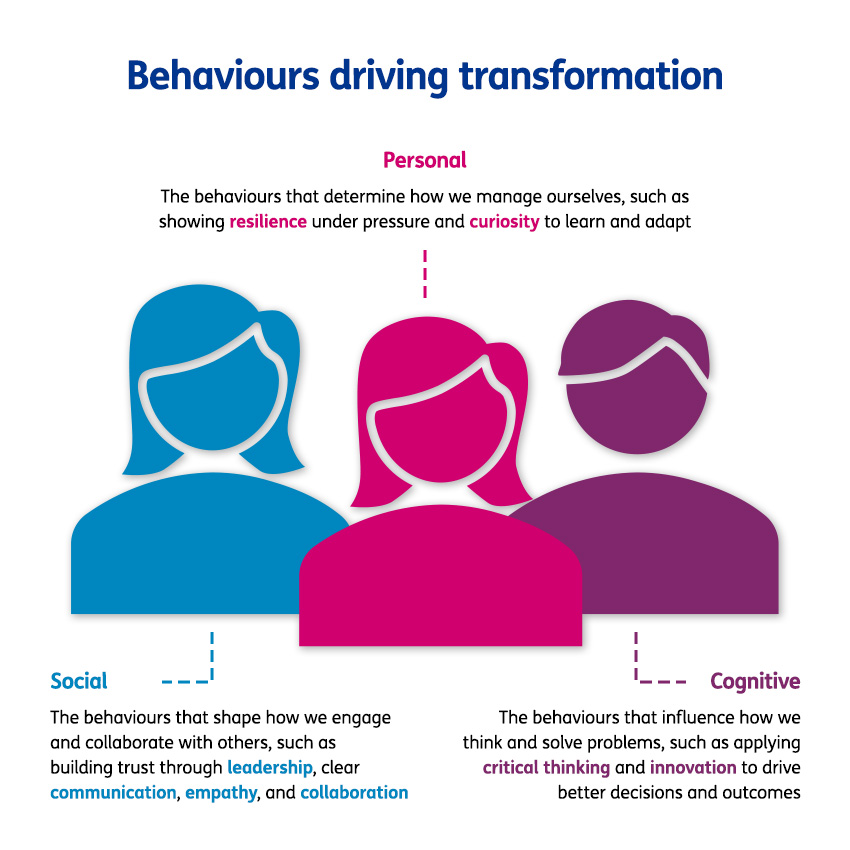
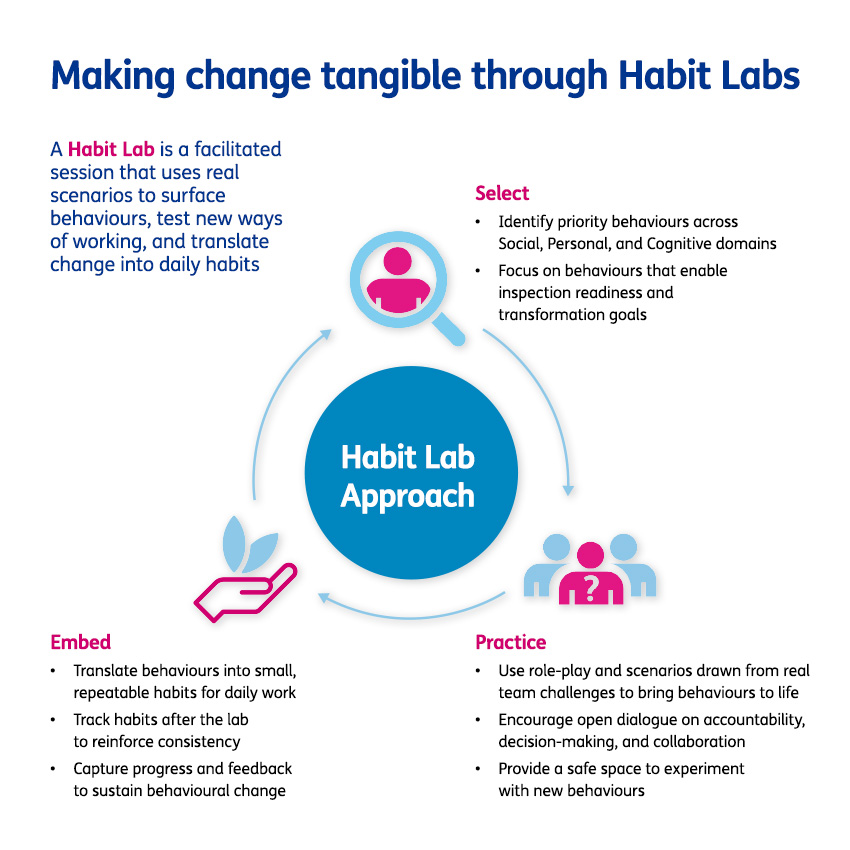
Driving rapid cultural change in a highly regulated GxP environment requires more than just traditional compliance training. It demands a sustained focus on the mindsets and behaviours that impact how people work every day. While quality is often perceived as a constraint in fast-moving, scaling organisations, the reality is that strong quality foundations enable speed, confidence, and resilience.
Our client was entering a period of intensive regulatory inspections linked to the progression of assets into Phase 3. They were continually being faced with the challenge of balancing an ability to move at pace with the demands of maintaining a consistent state of high-quality operations and inspection readiness.
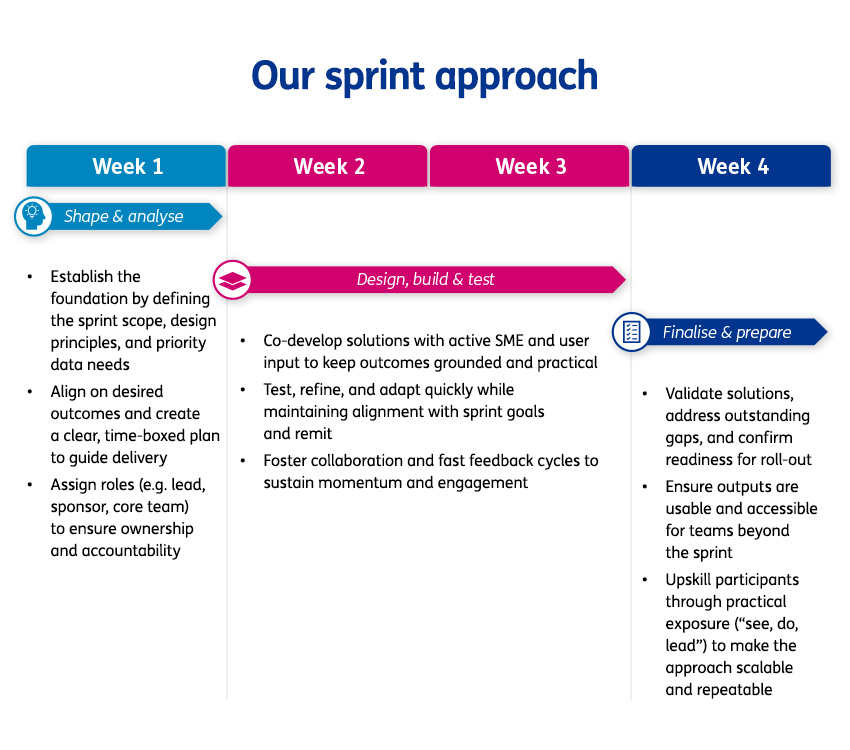
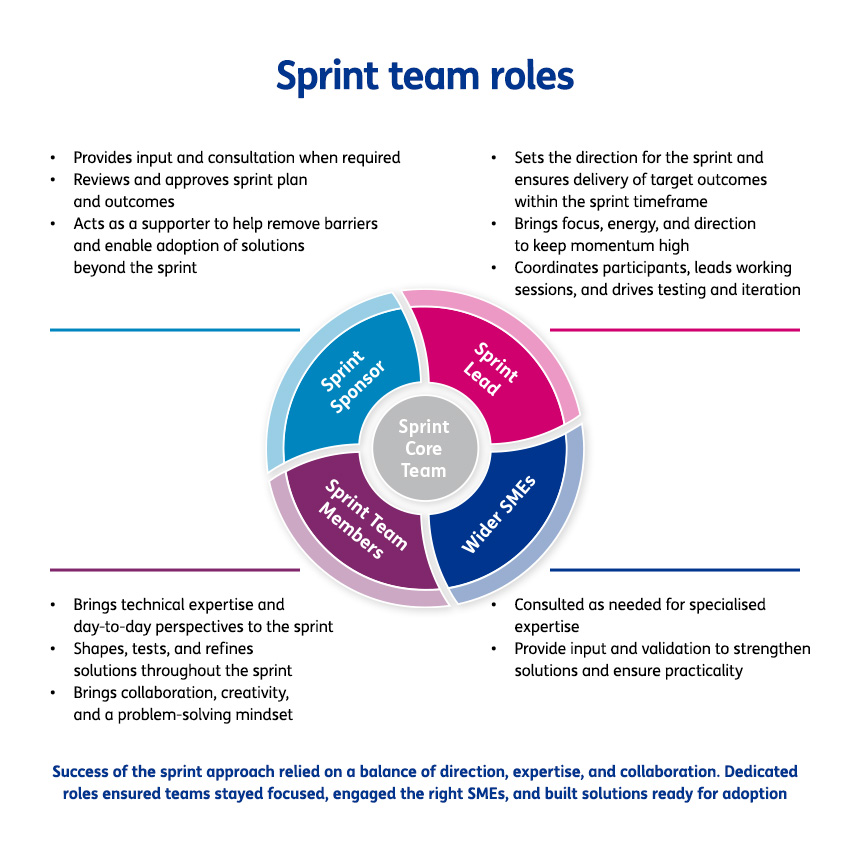
To address this challenge, the organisation launched a transformation programme which targeted capability development, and lay the foundation for a quality-first culture built on ownership, cross-functional trust, and continuous improvement.
With a quality-first operating model now in place, the focus turns to scaling this mindset across adjacent functions, deepening capability through ongoing learning, and evolving processes to meet the demands of a fast-moving, regulated environment. The next chapter is not just about maintaining momentum, it is about unlocking the full potential of a culture where quality is not only expected, but owned.
Our Experts
Related Insights

AI in healthcare needs more than hype – it needs quality
Digital and AI innovation is rapidly propelling the healthcare and life sciences industry into a new technological era, where the art of the possible is being continuously reimagined, creating more opportunities to unlock value and ultimately drive better health outcomes for patients.
Read more
Reimagining a critical R&D process through AI
A top 10 global biopharmaceutical company partnered with Baringa to address a challenge familiar across R&D.
Read more
Transformation of QMS: Why evolution is inevitable and urgent
The pharmaceutical industry is experiencing a rapid acceleration in the adoption of artificial intelligence (AI), automation and advanced digital technologies across R&D.
Read more
Enabling commercial agility in medical device manufacturing
A global medical device company was operating with limited visibility across its commercial and operational functions.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?