
The NHS Covid-19 vaccine supply chain delivers a 'shot in the arm' for the UK
19 July 2021
I wrote my last blog #buildbackbetter: the post-Covid supply chain of the future almost exactly a year ago, as the world headed into the unchartered territory of the Covid pandemic and caused supply chains to change almost overnight. This next instalment starts in September 2020, en route to a family holiday in Wales, and just weeks before ‘lockdown 2’ descended on the UK.
Earlier that year, a team of Baringa colleagues had supported the Government’s supply chain response to the PPE [1] crisis. At the time the project started, my wife and I were expecting our third child with a sense of fear and trepidation as we watched hospitals in Italy creak under the strain of Covid. With a heavy heart, we made the decision as a family that I would not join the project a decision I wondered if I would end up regretting!
Wind the clock forward to September en route to Wales to our first family holiday of five - our son had arrived safely a couple of months earlier. Following my colleagues’ earlier work on PPE and Test & Trace, we were now invited to support the NHS with designing and building the Covid vaccine supply chain. This one I couldn’t pass up. As I struggled for reception through a phone interview with the NHS program lead, the sense of anticipation grew palpable. Next day, Baringa was asked to start work as soon as possible. We cut short the holiday, packed and moved out of London for more space as we headed into lockdown 2. I caught up with colleagues who had been working on the earlier programs about what to expect.
Nothing could prepare me for what was to follow.
We inherited two of the program’s key workstreams: EECL (Estates, Equipment, Consumables & Logistics) and Commercial – effectively the supply chain and procurement functions of the NHS Covid Program. Both faced challenges: uncertainty on the vaccine handling requirements impacted the supply chain design, and billions of non-vaccine supply items (PPE, equipment and consumables) needed to be sourced compliantly at pace. On top came the uncertain start of the vaccine-rollout which depended on the progress through late phase clinical trials and the unprecedented regulatory approval process. This made for a tricky start to of the program.
A fateful week in October turned our world on its head. Firstly, the full Primary Care Network (GPs) was brought into scope for the vaccination rollout: an additional 1,500 drop points on top of the ~500 Hospital Hubs and Vaccination Centres we had been planning for. Secondly, MHRA [2] and Pfizer outlined a path through conclusion of phase 3 trials and regulatory approval, setting an ambitious start date for the vaccination rollout of early December. And finally, late stage trials confirmed challenging vaccine handling requirements. In short, we needed to redesign the supply chain, which had tripled in size, with five weeks to go-live!
Matt Hancock took to the Number 10 lectern that week and promised the country “the NHS will be ready”. The nation was waiting, and our race was on.
The team and I redesigned the most important supply chain we will ever build in our lifetimes - in three days! A key decision we made early on was to separate vaccine and non-vaccine supply chains and run them independently from the NHS BAU supply chain, which was already under significant pandemic response strain and heading into its regular winter peak. We awarded vaccine logistics contracts to two medicines wholesalers after a short compliant tender process, with the intent to leverage their existing scale, spare capacity and medicines distribution and processing licenses as they completed the regular flu campaign. For the non-vaccine supply chain, warehouse availability was a challenge as the impending Brexit deal had consumed most of the spare capacity available in the UK. Eventually, our sourcing process came good and we completed the supply chain design by combining a leading 3PL-provider [3], with spare logistics capacity from the NHS Supply Chain (SCCL [4]) BAU operation, and the PPE network. Designed in three days, built in five weeks - an unprecedented supply chain for unprecedented times. But would it work?
The first test was whether we would be able to deliver the Pfizer vaccine to care homes in the opening weeks of the program to save lives in one of the most vulnerable and hard-hit cohorts, rather than waiting for the deployment of the AZ vaccine early in the new year. A tricky test given the fragility and transport restrictions of the Pfizer vaccine. A rapid supply chain design undertaken with NHS strategy and clinical colleagues was followed by equally quick sourcing of specially designed medical grade cool boxes. We piloted to a handful of selected sites and were ready for go live.
And then it happened. The whole team watched the breakfast news on 8 December 2020 to see Margaret Keenan receive the first publicly administered dose of the Pfizer vaccine globally in Coventry. After 12 unforgettable weeks when I barely saw my young family and feeling the pressure from the huge public interest in the program, I am not ashamed to say tears rolled down my cheeks that morning, as they did for many others across the program.
We came back from a short Christmas break with a sense of achievement, but the job was only half done. We quickly trained our sights on government’s mid-April target to complete vaccination of cohorts 1 to 9 [5]. The rollout of second doses also needed to be planned for, and the various changes to the dosing interval were signs of the volatility and uncertainty to come. What followed through the spring tested the resilience and agility of our newly designed and built supply chain to the limit.
The first test was dealing with supply volatility caused by immature manufacturing processes and the dependency on international shipments. Our demand profile was equally tricky: strong early demand surpassed expectations but was followed by a softening of demand as society reopened and younger cohorts were invited to come forward for vaccination. In between we had to incorporate changes to guidelines for the under 40s, and then for the under 30s who should no longer receive the AZ vaccine – relatively straightforward rulings, but with huge impact on operational complexity. On top there were new vaccines to deploy, snow disrupting transportation, and surge vaccination requests to combat the Delta variant – every week seemed to bring a new challenge!
Yet this immature supply chain built in five weeks stood up to everything thrown at it. To date we have delivered over 80m vaccines and hundreds of millions of non-vaccine items to over 2,000 sites, a larger network than most - if not all - UK retailers. We have done this with an on time and in full delivery (OTIF) rate in excess of 99%; damages and losses are almost non-existent. Public Health England (PHE) estimate that the Covid vaccine program has saved more than 30,300 lives, prevented 46,000 hospitalisations and 8.2m infections. The program estimates it was saving a life a minute at the height of the pandemic in January. An incredible achievement! The supply chain was undoubtedly one of the key contributors to the success of the program.
As we reflect on our accomplishments to date and take the learnings into planning for the autumn vaccination ‘booster’ campaign, it struck me that the Post Covid supply chain of the future that I wrote about last year has strong parallels with our rapidly built vaccines-supply chain:
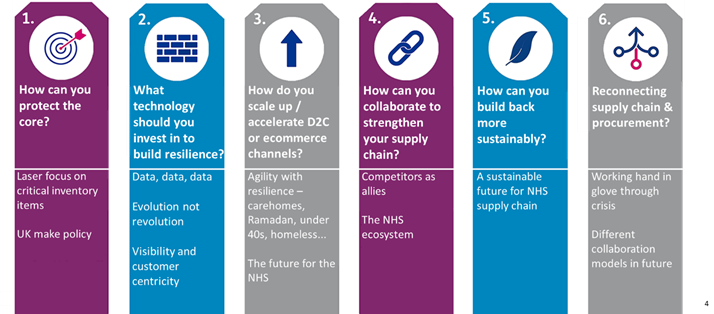
Protecting the core: The UK vaccines program has done a fantastic job of protecting its core, the most critical items needed for deployment, allowing the rollout to continue at pace despite all the challenges thrown at the supply chain. The PPE supply chain already focused heavily on ‘Made in UK’ and the program continues to work closely with the Vaccines Task Force (VTF) to ensure the booster campaign demand will be met - wherever possible - with UK-supplied vaccines to improve supply certainty.
Technology: Technology has undoubtedly been a key enabler of the success of the program – several politicians commended the program for having more data at its fingertips than any other government program in history. The data analytics platform has blown open visibility of the end-to-end supply chain, with particular focus on track and trace. This enables vaccination sites to understand their planned delivery times and allows them to schedule time-critical clinics [6].
Scale up and D2C: The vaccine supply chain is the most extensive and agile ever developed by the NHS, reaching far beyond the traditional hospital network to thousands of vaccination centres, GPs and community pharmacies. Our ‘roving models’ [7] out from these sites have enabled a direct to consumer (patient) service to tackle some of the hardest challenges the program has faced including evening clinics at mosques during Ramadan, vaccine buses to provide surge vaccinations for the Delta variant and roving teams targeting hard to reach groups such as the homeless and traveller community, and the initial care home challenge.
Collaboration: Collaboration has been at the heart of the supply chain with five different logistics companies combining to operate the supply chain. More widely, the NHS ecosystem across the centre, regions and sites worked together in a way not seen before, offering the scale and reach that were critical to the speed of the program. This is the NHS at its best; something we should be truly proud of.
A sustainable supply chain: Thoughts are now turning to how to integrate the Covid-supply chain back into the NHS’s BAU supply chain. The legacy of the program in terms of patient reach, data visibility and how to get the best from 3rd parties bode well for a more agile and resilient NHS supply chain that’s ready to withstand future pandemics and operate BAU in a more sustainable way.
Connecting supply chain & procurement: And finally, the supply chain and procurement functions worked together seamlessly through this program, stitching together an end-to-end supply chain that is lean and cost efficient as well as agile and resilient.
The UK is well on track to meet its target of offering a first Covid vaccine to all adults by the end of July. As we celebrate Freedom Day on July 19 and head into a brighter post-Covid future, it is clear the NHS has not only saved thousands of lives, but also given the UK a ‘shot in the arm’ with an improving economic outlook and sense of national pride. And if the NHS can deliver an agile and resilient supply chain built in a few short weeks, it offers hope for others looking to develop a post-Covid supply chain of the future!
Reference list:
1. Personal protective equipment such as gloves, masks, scrubs and aprons
2. Medicines and Healthcare products Regulatory Agency
3. Third-party logistics provider
4. Supply Chain Coordinated Limited, a UK company that acts as the management function of the NHS Supply Chain.
5. Cohort one to nine were the groups prioritised for the Covid-19 vaccine: care home residents and staff, over 80s, frontline health and care staff, over 75s, over 70s, clinically extremely vulnerable people, over 65s, under 65s with underlying health conditions, over 60s, over 55s, over 50s.
6. Note: the Pfizer vaccine must be used in under 3.5 days after the thawing process has started due to the short shelf life of the active ingredient
7. The roving models enable the administration of Covid-19 vaccines at identified locations outside of vaccination base sites (i.e. vaccination centres, hospital hubs, PCN-led sites and community pharmacies) and includes care homes, temporary vaccination clinics and pop-up sites, vaccination buses, drive-through clinics and at home for the housebound.
Related Insights

From funding to fulfilment: building confidence in public sector digital and AI transformation
Digital promises better outcomes, but many efforts fall short. How can public leaders ensure AI programs are set up for success and lasting impact?
Read more
Embedding innovation in government to drive continuous improvement and optimisation
In a world of constant technological change, innovation is no longer optional—it's essential. This video explores how public sector organizations can rise to meet citizens' growing expectations by embedding innovation as a core capability.
Read more
Shifting the paradigm from projects to products – Lessons from UK Home Office
Who is responsible for evolution of the services and digital products created by programmes that have ended? A question faced to all Government departments as they try to embed the principles set out in the Blueprint for a Modern Digital Government.
Read more
How can you ensure digital and AI keeps delivering real-world outcomes once it’s live for long-term impact?
So how do you ensure AI keeps delivering real-world outcomes once it’s live? We break down a practical, people-focused approach to continuously optimise performance in BAU—keeping people, process, and tech moving together.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?