Optimising global R&D processes to accelerate drug approval
31 October 2025
For large pharmaceutical companies, the ability to consistently deliver high-quality medicines to patients as quickly and efficiently as possible is a critical differentiator. A streamlined and effective Quality Management System (QMS) is essential in achieving this goal, enabling regulatory compliance, improving operational efficiency, and ensuring quality is embedded from the outset of drug development.
How can a global pharmaceutical company harmonise and simplify R&D processes to drive regulatory efficiency and accelerate delivery?
Faced with the challenge of accelerating regulatory approval for high-quality medicines, a leading global pharmaceutical company’s Development Leadership Team sought to harmonise and simplify its ways of working across more than 90 countries. The complexity of operating in diverse markets, each with unique regulatory demands, had resulted in overly complex and duplicative processes, inconsistent documentation, and a lack of standardisation - creating inefficiencies and unnecessary risk across R&D. By focusing on global process harmonisation, with localisation only where required by local regulations, we helped the client address these challenges head-on, laying the foundation for streamlined, compliant, and efficient operations that enable faster, high-quality delivery.
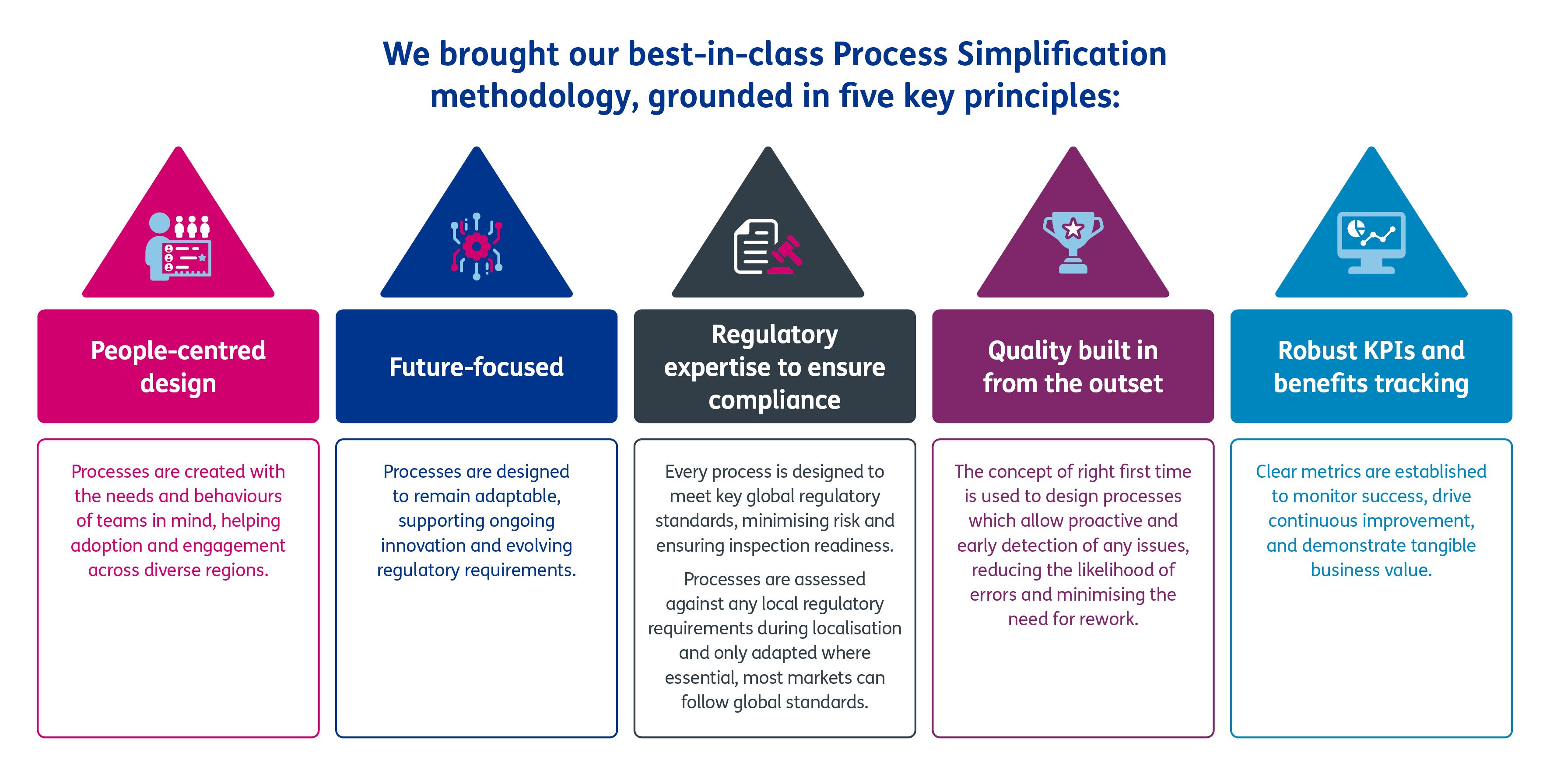
How did we empower our client to achieve their objectives?
Click to enlarge
Working hand-in-hand with cross-functional teams, we mapped and re-designed 60 end-to-end R&D processes.
We went beyond process design by implementing four key success factors.
- We helped the client to pivot away from unengaging and ineffective training which typically only involved “Read and Understand” of the procedural document to engaging training which brought to life the process documentation by providing real scenarios and guiding the learner through why this is important but also how to do it well.
- We created a new process governance structure which gave a single Global Process Owner the accountability for each process and the empowerment to make decisions and monitor progress effectively.
- Over the two years of the project we created a dedicated communications campaign, which included the creation of a repository to act as the single source of truth for all R&D process documentation and a regular newsletter to update the entire company on what had been achieved to date and what was coming next.
- Finally, by embedding new mindsets and behaviours alongside new processes, we left the company primed for continuous improvement and global alignment.
What was achieved?
The results were significant. We helped the client optimise and harmonise 60 global R&D processes, ensuring they were regulatory compliant, generated high-quality outputs, and enabled flexible ways of working.
The streamlined approach reduced the global document landscape by over 20%, cutting complexity and improving efficiency.
Our work with the client also contributed to the acceleration of their cycle times resulting in an improvement from bottom to top-quartile of industry peer performance within 3 years.
Most importantly, the client now has a consistent and scalable foundation to support accelerated drug discovery and approvals worldwide. By combining technical expertise with a people-centred approach, we ensured sustainable adoption - positioning the organisation to deliver innovation to patients faster, with quality and compliance at its core.
"Baringa bring their vast knowledge to the team without ego, to truly act as a partner with a vested interest in achieving the company’s goal(s), while building trusted relationships along the way. Baringa have been flexible, nimble and reasonable when planning complex workshops and have conducted the workshops, as well as the related post work, with masterful expertise, brave candour and even some humour too!"
VP, Global Regulatory Platforms & Systems
Related Client Stories

Reimagining a critical R&D process through AI
A top 10 global biopharmaceutical company partnered with Baringa to address a challenge familiar across R&D.
Read more
Enabling commercial agility in medical device manufacturing
A global medical device company was operating with limited visibility across its commercial and operational functions.
Read more
A focused integrated evidence planning approach that identified over >£10m of savings in one year
Our client was a leading global pharmaceutical company with an ambitious 5-year pipeline of launches that required a cross-functional step-change in how they approached evidence generation.
Read more
Preparing a global biotech company for inspection readiness
Always being inspection ready is integral to maintaining an effective Quality Management System (QMS), ensuring regulatory compliance, safeguarding the quality and safety of pharmaceutical products, and reducing risks and issues.
Read moreIs digital and AI delivering what your business needs?
Digital and AI can solve your toughest challenges and elevate your business performance. But success isn’t always straightforward. Where can you unlock opportunity? And what does it take to set the foundation for lasting success?